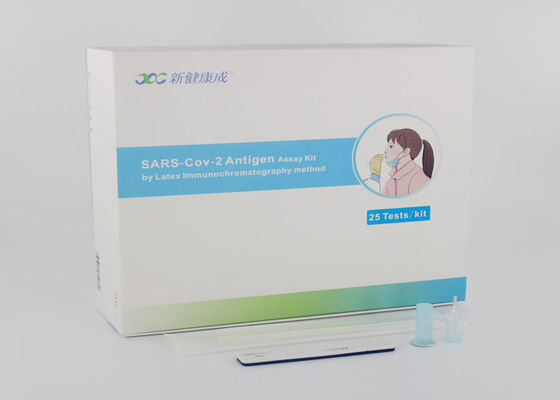
15 mins No Instrument Covid-19 Test Kit/SARS-CoV-2 Antigen Assay Kit by Latex Immunochromatography Method
Test Principle:
Latex immunochromatography method is used to detect SARS-Cov-2, influenza A or influenza B virus antigens. After treatment when SARS-Cov-2, influenza A or B virus antigens in the sample are added to the detection hole, the antigen and latex labeled antibody (monoclonal antibody against SARS-Cov-2, influenza A or B antigen) form an immune complex. The complex moves with the liquid to the nitrocellulose membrane, and the detection area of the nitrocellulose membrane is coated with another one.
Test Result:
When monoclonal antibody is against SARS-Cov-2, influenza A or B antigen, the immune complex is captured by the antibody on nitrocellulose membrane, forming a red reaction line: the result is positive. When the sample does not contain SARS-Cov-2 antigen, influenza A or B antigens or are lower than the minimum detection limit of this kit, no red reaction line appears in the detection area: the result is negative. At the same time, whether the result is positive or negative, as the internal control standard of reagents, a red reaction line will be formed in the quality control area (line C), which is used to judge whether the chromatographic process is normal or not.
In Conjuction with IgG/IgM Antibody Test
- Accuracy: the inaccuracy of antigen test due to manual sampling is addressed by antibody test, resulting in combined accuracy of close to 100%.
- Easy of Use: flexibility in determining either or both tests to apply given operator's availability.
- Speed: antibody test takes 8 min and both tests can be done in 15 min simultaneously.
- Early Detection: the deficiency of testing during incubation period of antibody test is supplemented by antigen test.
- Cost Effectiveness: the price of nucleic acid (PCR) detection weighs more than antibody and antigen tests combined, given PCR test is labor-intensive in operation and demanding with lab environment.
- Non-Laboratory Settings: both antibody test by colloidal gold and antigen are operated without instrument. Antibody test by immunofluorescence chromatography method needs a small instrument. No lab for analyzing or low temperature for preservation is required.
| Specification |
1or 5 or 25 tests/box |
| Methodology |
Latex Immunochromatography |
| Validity |
18 months
|
ABOUT US
Sichuan Xincheng Biological Co.,LTD (Diacegene as brand for overseas market), founded in 2007, is a global leading bio-technical company, dedicating to develop and manufacture In Vitro Diagnostic (IVD) products, providing"Analyzers & Reagents" and "Products & Services".
Sichuan Xincheng Biological Co., Ltd.(referred to as Xincheng BIO and Diacegene as brand for overseas sale), was founded in 2007, located in Chengdu High-tech Zone, Sichuan Province, P.R. China. It's a high-tech enterprise combined with R&D, production, sales of in vitro diagnostic (IVD) "instrument & reagent ", and provide" products & services ". The company was listed in the National Equities Exchange And Quotations(NEEQ) stock market on 2014, stock code 831193.
The company has a production base of 18.7 mu, building area of 24,000 m², the main products including automatic biochemical analyzer and supporting reagents, Point-of-care Testing(POCT) products, and more than 100 independent products. Xincheng BIO got quality management system certification of ISO9001,ISO13485, participates in the National Inter-room Quality Assessment Scheme to promote standardized traceability work.
As an in vitro diagnostic(IVD) products and services integrated program supplier, the future of Xincheng BIO will continue to adhere to the "customer-centered, teamwork, innovation, pragmatic, dedicated, progressive" core values, to be the innovator of IVD and the promoter of the general health care.




 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!