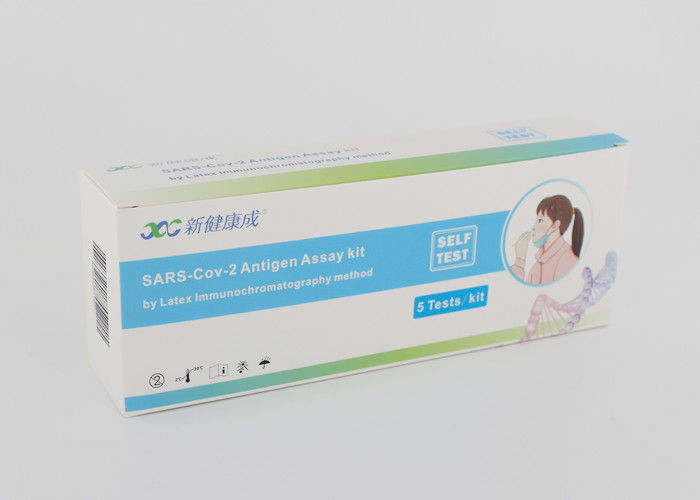
SARS-Cov-2 Antigen Assay Kit by Latex Immunochromatography Method Self-Testing Type
5 Tests Per Boxes, Great for Family Members
Summary
This product can be used as a supplementary test indicator for suspected cases with negative nucleic acid test of SARS-Cov-2 or used in conjunction with nucleic acid testing in the diagnosis of suspected cases. It cannot be used as a basis for the diagnosis and exclusion of pneumonia caused by SARS-Cov-2 infection.
Precautions and warnings
1. This product is for in vitro diagnostic only.
2. Please test in a quiet and independent scene such as home or hotel.
3. This product contains small parts, storage and testing process should avoid contact with infants and children.
4. Perform test at room temperature 15 to 30°C.
5. Ensure foil pouch containing test device is not damaged before opening for use.
6. Do not use after the expiration date.
7. Test cards, swabs, pipettes, etc. are for single use.
8. Wear gloves when handling samples to avoid contact with the reagent membrane and the sample (observation) area.
9. Do not mix components with different batches or different brands of in vitro diagnostic reagents.
10. To obtain accurate results, do not use samples that are too viscous or bloody. 11.Process in accordance with the Specimen collection and Sample extraction methods in this manual. Failure to follow the instructions for use may result in inaccurate test results.
Materials required but not provided
1. Timer (or stopwatch, clock)
2. Protective gloves
Interfering substances
When hemoglobin ≤ 5g/L, bilirubin ≤ 342.0 μmol/L, triglyceride ≤ 11.3mmol/L, rheumatoid factor ≤ 80IU/ml, there was no significant effect on the test results.
| Methodology |
Latex Immunochromatography |
| Validity |
18 months |
| Sample Type |
Nasopharynx/oropharyngeal swab collection
|


 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!